
Centers for Disease Control and Prevention
Center for Preparedness and Response
Johnson & Johnson/Janssen COVID-19 Vaccine and
Cerebral Venous Sinus Thrombosis with
Thrombocytopenia – Update for Clinicians on Early
Detection and Treatment
Clinician Outreach and Communication Activity (COCA) Webinar
Thursday, April 15, 2021
To Ask a Question
▪ All participants joining us today are in listen-only mode.
▪ Using the Webinar System
– Click the “Q&A” button.
– Type your question in the “Q&A” box.
– Submit your question.
▪ The video recording of this COCA Call will be posted at
https://emergency.cdc.gov/coca/calls/2021/callinfo_041521.asp and available to view
on-demand a few hours after the call ends.
▪ If you are a patient, please refer your questions to your healthcare provider.
▪ If you are a member of the media, please contact CDC Media Relations at 404-639-3286, or send an
email to media@cdc.gov.
▪ Closed-captioning will not be available during today’s webinar. A transcript and closed-captioned
video will be posted to the COCA Call page located at
https://emergency.cdc.gov/coca/calls/2021/callinfo_041521.asp as soon as possible after today’s live
session.
Today’s Presenters
▪ Tom Shimabukuro, MD, MPH, MBA
CAPT, U.S. Public Health Service
Vaccine Safety Team Lead
COVID-19 Response
Centers for Disease Control and Prevention
▪ Sara Oliver, MD, MSPH
LCDR, U.S. Public Health Service
Co-lead, Advisory Committee for
Immunization Practices COVID-19 Vaccines
Work Group
COVID-19 Response
Centers for Disease Control and Prevention

National Center for Immunization & Respiratory Diseases
Reports of cerebral venous sinus thrombosis with
thrombocytopenia after Janssen COVID-19 vaccine
Clinician Outreach and Communication Activity (COCA)
April 15, 2021
Tom Shimabukuro, MD, MPH, MBA
CDC COVID-19 Vaccine Task Force
Vaccine Safety Team

5
Disclaimer
▪ The findings and conclusions in this report are those of the authors
and do not necessarily represent the official position of the Centers for
Disease Control and Prevention (CDC) or the U.S. Food and Drug
Administration (FDA).
▪ Mention of a product or company name is for identification purposes
only and does not constitute endorsement by CDC or FDA.

6
Topics
▪ Background
▪ Reports of cerebral venous sinus thrombosis (CVST)
with thrombocytopenia (low platelets) following
Janssen COVID-19 vaccine
▪ Summary

Background

8
Platelets and thrombocytopenia (low platelets)*
▪ Platelets (thrombocytes) are colorless blood cells that help blood clot;
normal platelet count is 150,000–450,000 per microliter
▪ Platelets stop bleeding by clumping and forming plugs in blood vessel
injuries
▪ Thrombocytopenia is a condition in which you have a low blood
platelet count (<150,000 per microliter)
▪ Dangerous internal bleeding can occur when your platelet count falls
below 10,000 platelets per microliter
▪ Though rare, severe thrombocytopenia can cause bleeding into the
brain, which can be fatal
* Source: https://www.mayoclinic.org/diseases-conditions/thrombocytopenia/symptoms-causes/syc-20378293
10
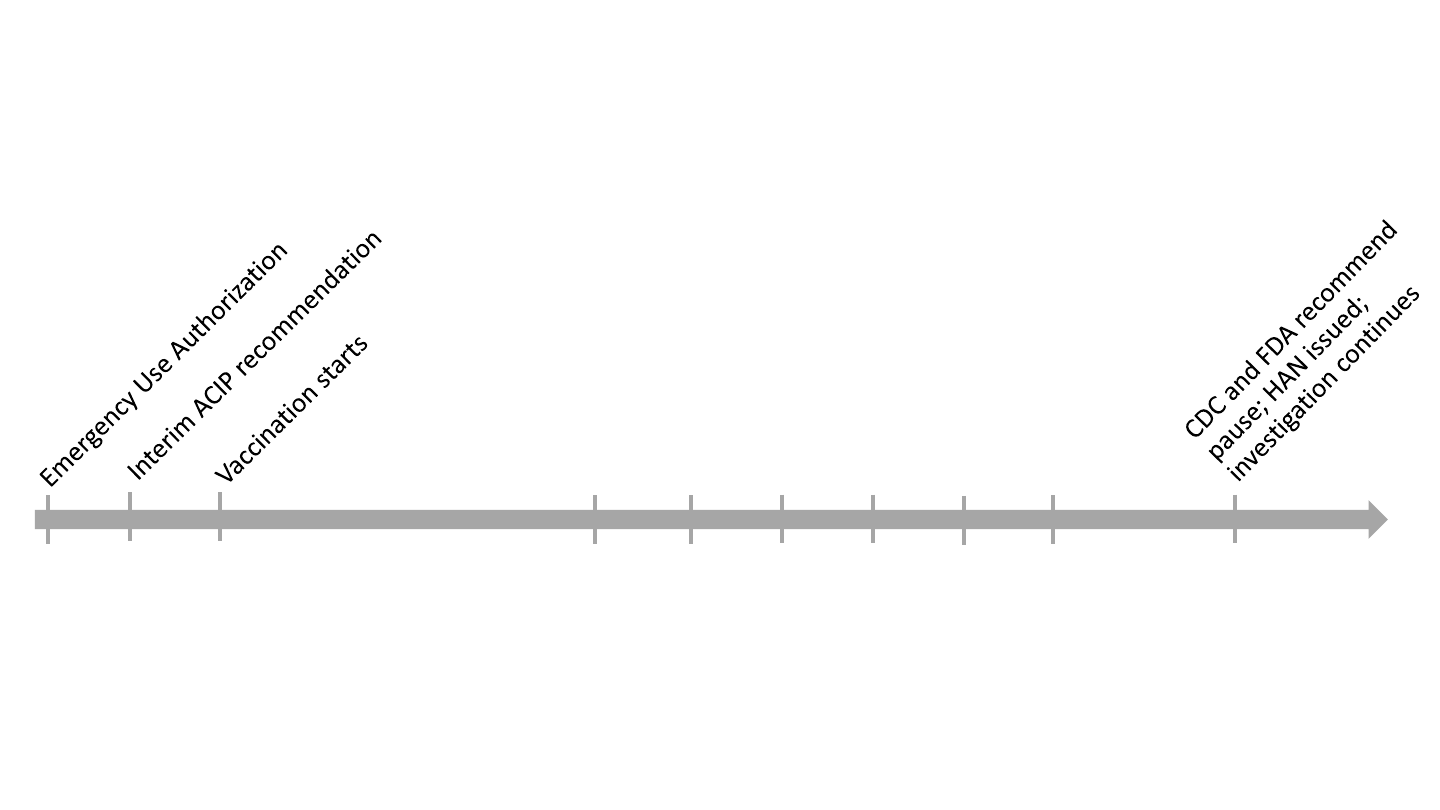
Janssen COVID-19 vaccine timeline* (2021)
6 CVST
†
with thrombocytopenia cases reported to VAERS;
records collection and investigation by CDC and FDA
Feb
27
Feb
28
Mar
2
Apr
13
Mar 19 thru Apr 12
* For illustrative purposes, not drawn to scale,
†
cerebral venous sinus thrombosis
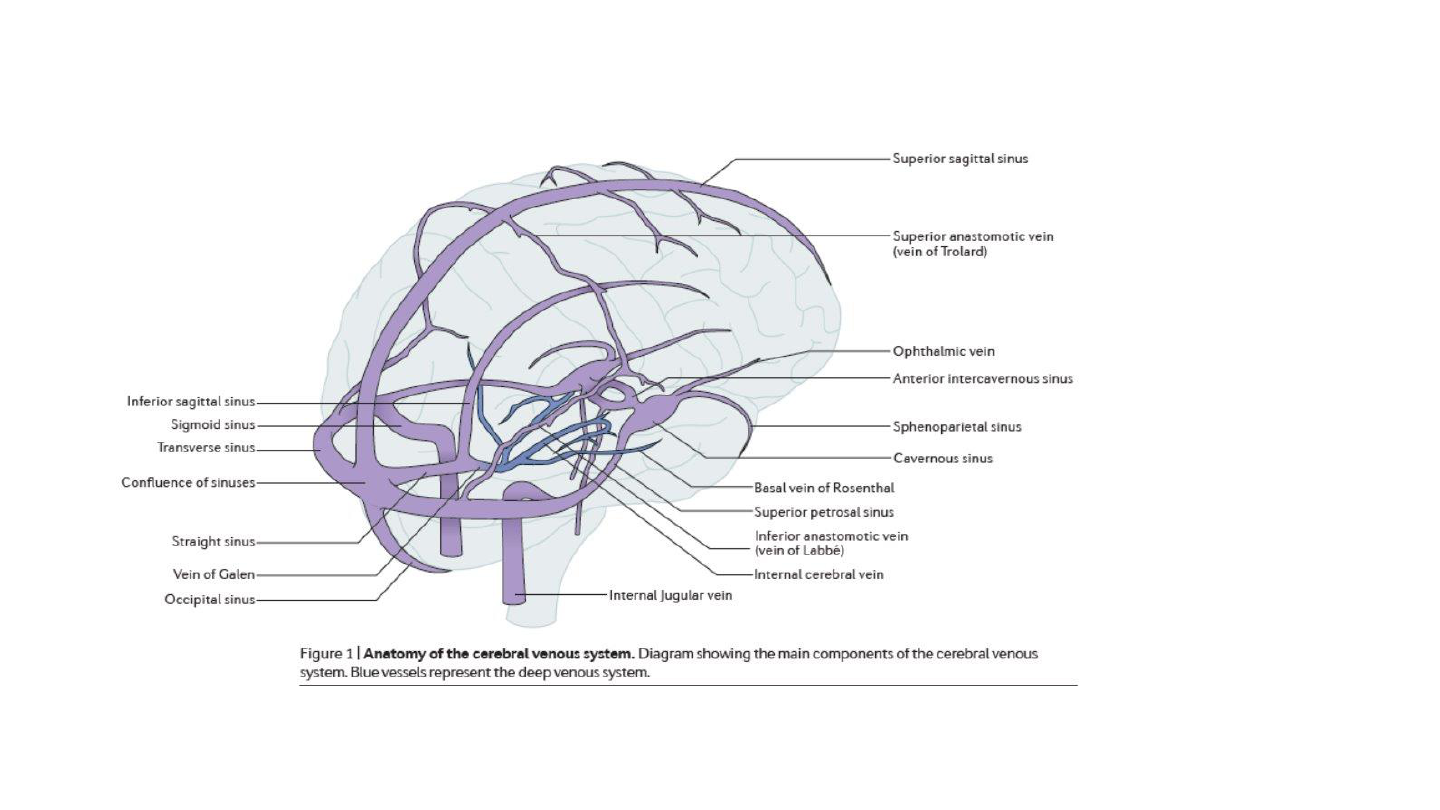
12
Cerebral venous sinus anatomy
Silvis SM et al, Nature Reviews Neurology 13, 555-565(2017)

13
Cerebral venous sinus thrombosis (CVST)
Background epidemiology
1-3
▪ Rare, 0.22–1.57 per 100,000,
~0.5-1% of all strokes
▪ Median age 37 years
▪ 8% of patients >65 years
▪ Female:male ratio of 3:1
Risk factors
4
▪ Prothrombotic conditions (genetic or acquired)
▪ Oral contraceptives
▪ Pregnancy and the post-partum period
▪ Malignancy
▪ Infection
▪ Mechanical precipitants (lumbar puncture)
1
Cerebral vein and dural sinus thrombosis in Portugal: 1980-1998. Ferro JM, Correia M, Pontes C, Baptista MV, Pita F, Cerebral Venous Thrombosis Portuguese Collaborative Study Group (Venoport) Cerebrovasc Dis. 2001;11(3):177.
2
The incidence of cerebral venous thrombosis: a cross-sectional study. Coutinho JM, Zuurbier SM, Aramideh M, Stam J. Stroke. 2012 Dec;43(12):3375-7..
3
Cerebral Venous Sinus Thrombosis Incidence Is Higher Than Previously Thought: A Retrospective Population-Based Study. Devasagayam S, Wyatt B, Leyden J, Kleinig T. Stroke. 2016 Sep;47(9):2180-2.
4
Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Saposnik G, et al. 2011;42(4):1158.

14
CVST signs and symptoms
▪ More common presentations
– Isolated intracranial hypertension syndrome (headache with or without
vomiting, papilledema, and visual problems)
– Focal syndrome (focal deficits, seizures, or both)
– Encephalopathy (multifocal signs, mental status changes, stupor, or coma)
▪ Rare presentations
– Cavernous sinus syndrome
– Subarachnoid hemorrhage
– Cranial nerve palsies

Data source and case reports

17
Reports of CVST to VAERS after COVID-19 vaccines
as of April 12, 2021
▪ Janssen COVID-19 vaccine
‒ 6 reports of CVST with thrombocytopenia (platelet counts <150K/mm
3
)
following 6.86 million doses administered
• Reporting rate of 0.87 cases per million doses administered
▪ Pfizer-BioNTech COVID-19 vaccine
‒ 0 reports following 97.9 million doses administered
▪ Moderna COVID-19 vaccine
‒ 3 reports following 84.7 million doses administered
‒ All 3 with normal platelet counts; onset 2, 6, and 12 days after vaccination
Source of doses administered: https://covid.cdc.gov/covid-data-tracker/#vaccinations

18
Reports of CVST to VAERS after COVID-19 vaccines
as of April 12, 2021
▪ Janssen COVID-19 vaccine
‒ 6 reports of CVST with thrombocytopenia (platelet counts <150K/mm3)
following 6.86 million doses administered
• Reporting rate of 0.87 cases per million doses administered
▪ Pfizer-BioNTech COVID-19 vaccine
‒ 0 reports following 97.9 million doses administered
▪ Moderna COVID-19 vaccine
‒ 3 reports following 84.7 million doses administered
‒ All 3 with normal platelet counts (150–450K/mm3)
Source of doses administered: https://covid.cdc.gov/covid-data-tracker/#vaccinations

19
Characteristics of patients with CVST and thrombocytopenia*
after Janssen COVID-19 vaccine, N=6
▪ Median age 33 years (range 18–48)
▪ Median time to symptom onset 8 days (range 6–13 days)
▪ All cases occurred in white females
▪ Current estrogen/progesterone use (n=1)
▪ Pregnant or post-partum (n=0)
▪ Pre-existing conditions
– Obesity (n=3)
– Hypothyroidism (n=1)
– Hypertension (n=1)
– Asthma (n=1)
– Coagulation disorders (none known)
* Note: Thrombosis usually does not occur in the
presence of low platelets; these case presentations
are atypical and consistent with cases observed
after AstraZeneca COVID-19 vaccine
20
Initial and late signs and symptoms among
CVST patients*, N=6 (patients listed in no particular order)
*All were hospitalized and admitted to the intensive care unit
Initial features Late features
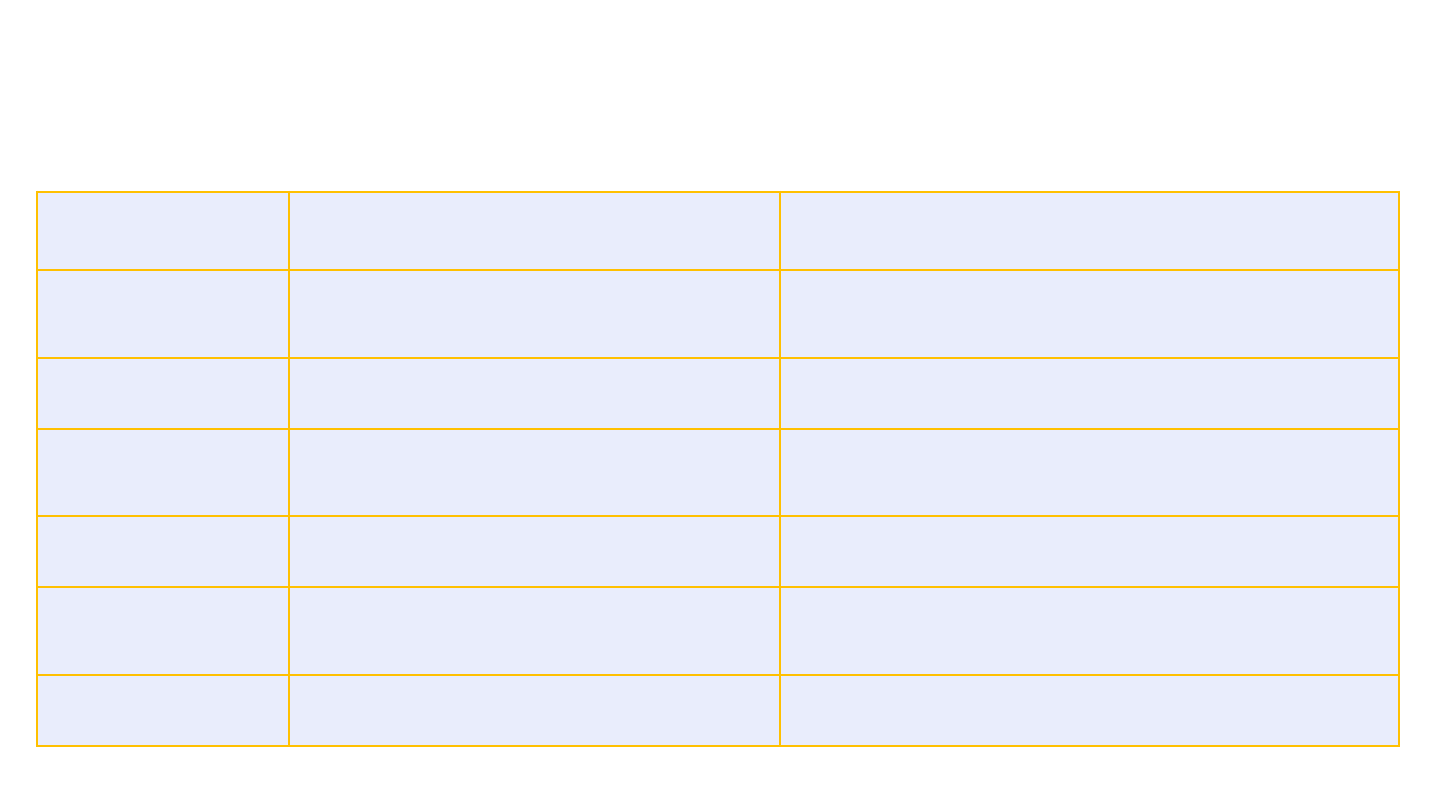
Patient 1
Headaches, lethargy
Severe headache, left
-sided weakness,
vomiting
Patient 2
Headaches
Severe headache, aphasia
Patient 3
Headaches, vomiting, fever
Left arm weakness, right gaze deviation,
left neglect
Patient 4
Headaches, chills, myalgias
Severe abdominal pain and fever
Patient 5
Headache, chills, dyspnea, fever
Bruising, unilateral leg swelling, loss of
consciousness
Patient 6
Back pain, bruising
Headache, abdominal pain
21
Locations of CVST, intracerebral hemorrhage, and
other thromboses, N=6
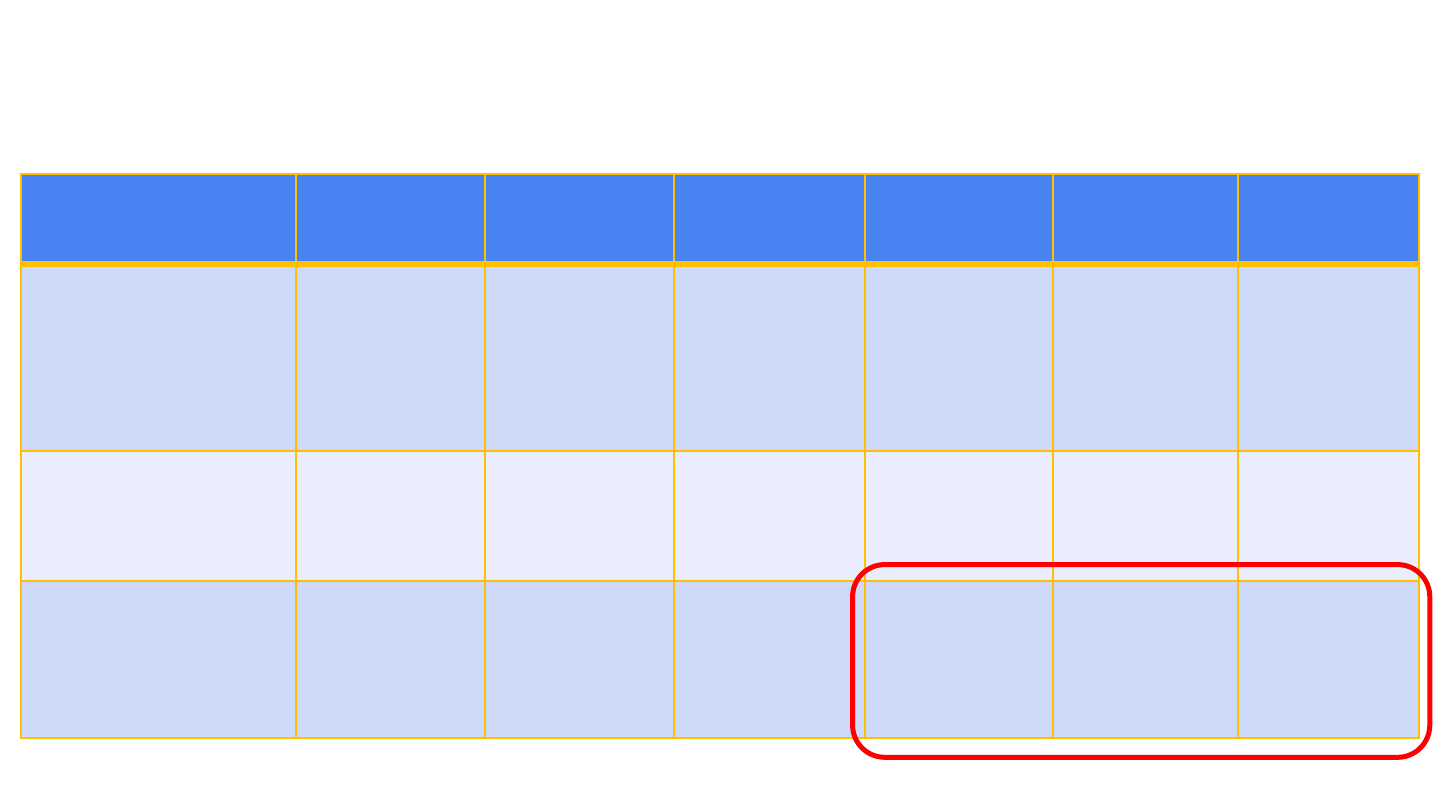
Characteristic
Patient 1
Patient 2
Patient 3
Patient 4
Patient 5
Patient 6
Location of CVST
Right transverse
sinus and right
sigmoid sinus
Left transverse
sinus, left
sigmoid sinus,
confluence of
sinuses, and
straight sinus
Superior sagittal
sinus, inferior
sagittal sinus,
and straight
sinus
Right transverse
sinus and right
sigmoid sinus
Right
transverse sinus
and right
sigmoid sinus
Right
transverse
sinus
Location of
intracerebral
hemorrhage
Right temporo-
parietal lobe
Left temporal
lobe
Bilateral frontal
lobes,
intraventricular
None None Occipital lobe
Locations of other
thromboses
None None None Portal vein and
right
pulmonary
artery
Bilateral lower
extremity VTE,
right internal
jugular vein
Portal vein
22
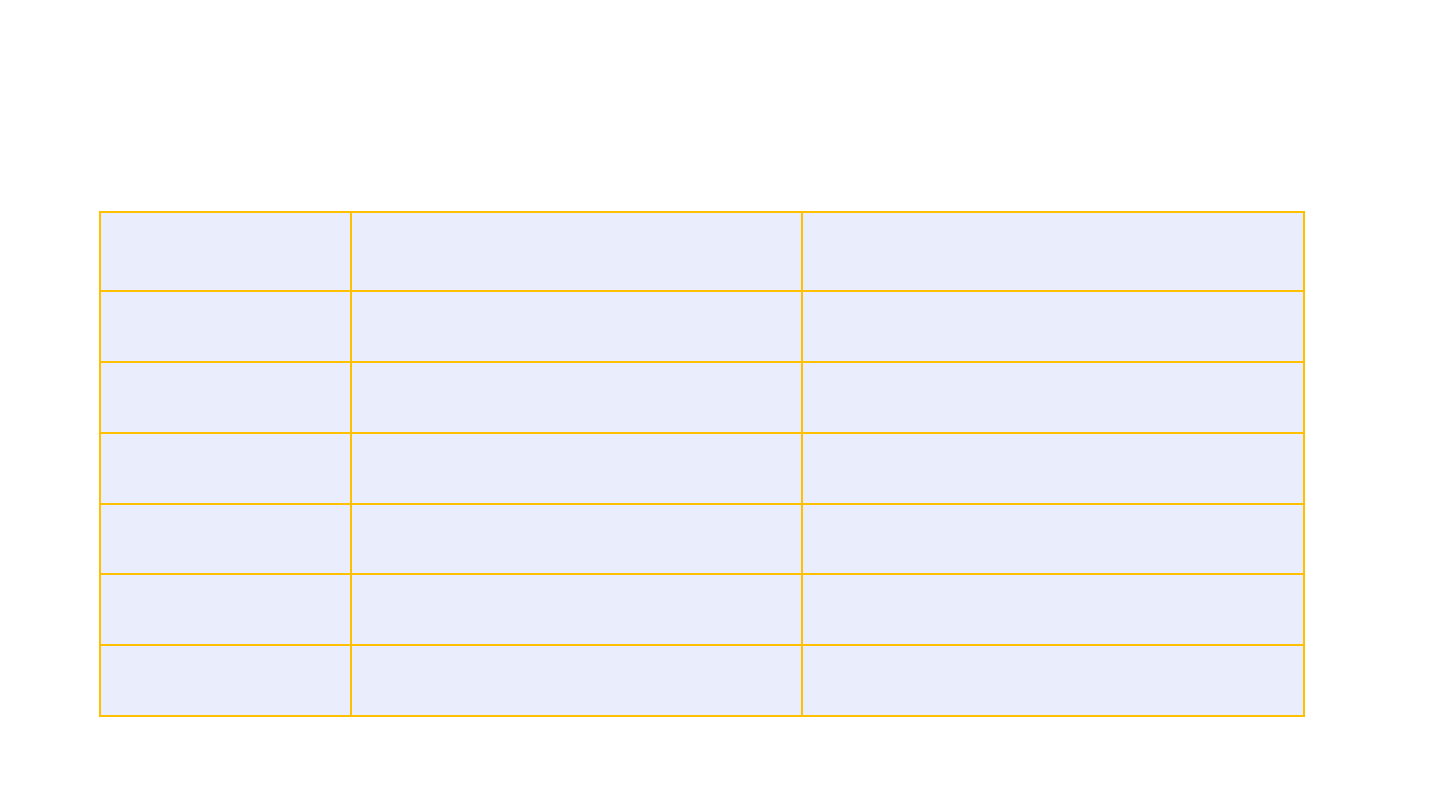
SARS-CoV-2 test results among CVST patients, N=6
SARS
-CoV-
2 viral test
SARS-CoV-2 serology
Patient 1 Negative
Not documented
Patient 2 Negative
Nucleocapsid Ab negative
Patient 3 Negative
Not documented
Patient 4 Negative
Not documented
Patient 5 Negative
Unspecified COVID Ab negative
Patient 6 Negative
Unspecified COVID Ab negative
23
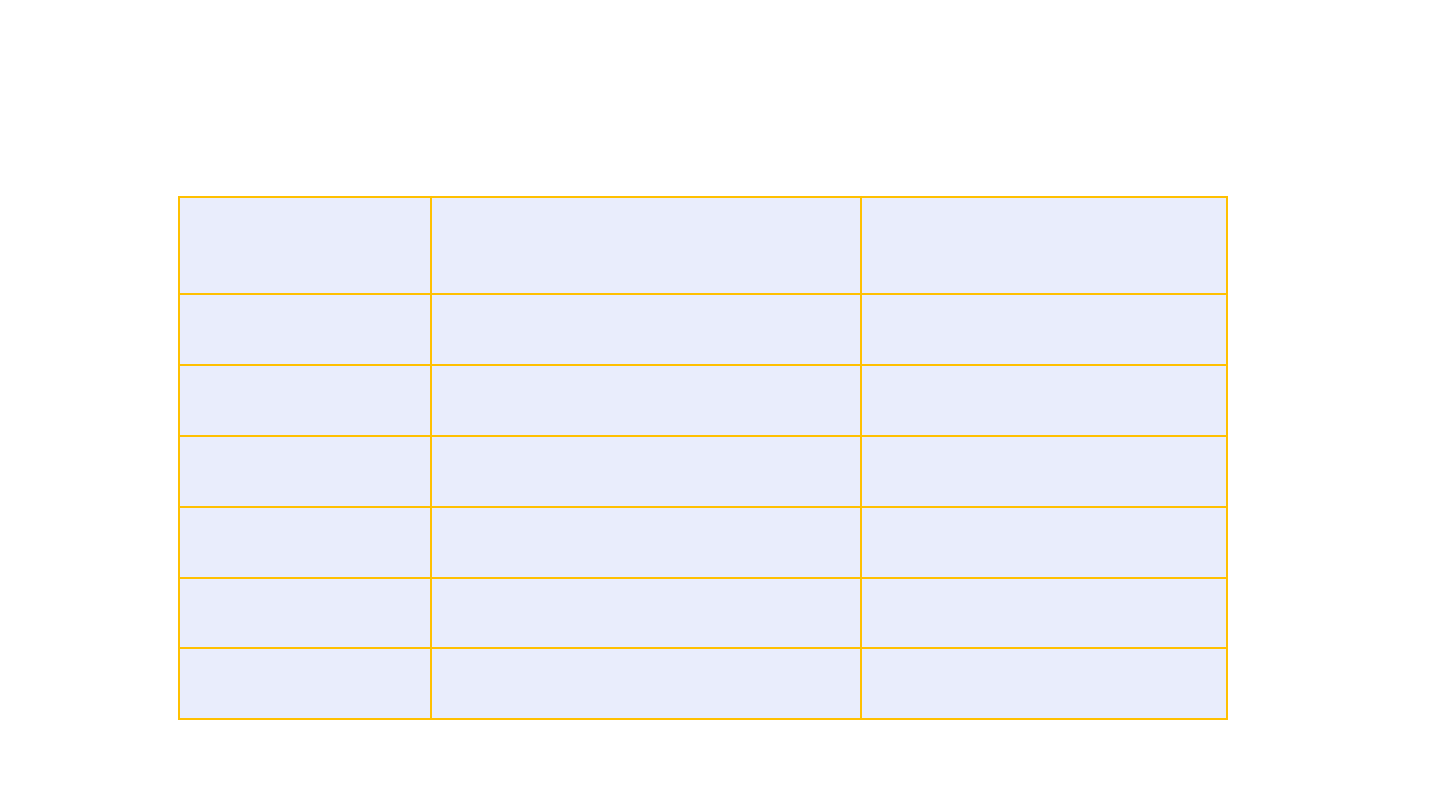
Hematology test results among CVST patients, N=6
*Platelet factor 4 heparin induced thrombocytopenia
Lowest platelet value
(per mm
3
)
PF4 HIT* antibody
test result(s)
Patient 1 12,000 Not done
Patient 2 69,000 Positive
Patient 3 18,000 Positive
Patient 4 127,000 Positive
Patient 5 10,000 Positive
Patient 6 14,000 Positive

24
Treatment and outcomes among CVST patients, N=6
▪ Treatment
‒ Heparin (n=4)
‒ Nonheparin anticoagulants (n=5)
‒ Platelets (n=3)
‒ Intravenous immunoglobulin (n=3)
▪ Outcomes
‒ Death (n=1)
‒ Remain hospitalized (n=3)
• Intensive care unit (n=2)
‒ Discharged home (n=2)
* All 5 of these patients received Argatraban
25
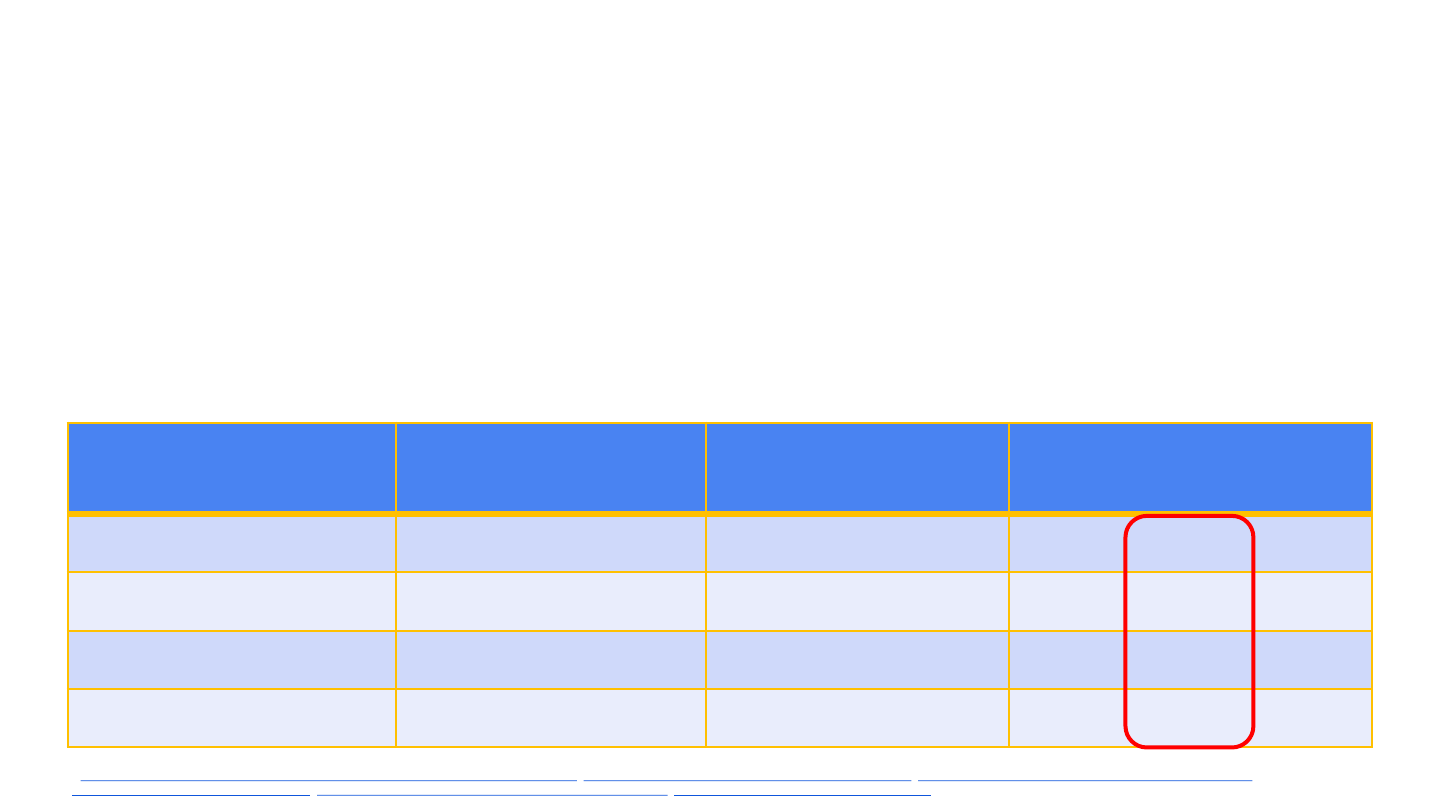
Observed vs. expected CVST cases following Janssen
COVID-19 vaccine
▪ Estimated annual incidence of CVST ~0.5–2 cases per 100,000 population*
▪ Assumed risk period of 5.6% of a calendar year: (41 days/2) ÷ 365 days
▪ Doses administered among women aged 20–50 years = 1,402,712 doses
(as of Apr 12)
Est. annual
background incidence
Obs. cases in women
aged 20–50 yrs
Exp. cases in women
aged 20–50 yrs
Reporting ratio, women
aged 20–50 yrs
0.5 per 100K 6 0.39 15.4
1.0 per 100K 6 0.79 7.6
1.5 per 100k 6 1.18 5.1
2.0 per 100k 6 1.58 3.8
* https://www.hopkinsmedicine.org/health/conditions-and-diseases/cerebral-venous-sinus-thrombosis, http://www.med.umich.edu/1libr/Stroke/SinusVeinThrombosis.pdf, https://www.nejm.org/doi/10.1056/NEJMra042354?url_ver=Z39.88-
2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub, https://www.ahajournals.org/doi/pdf/10.1161/STROKEAHA.116.013617, https://www.nature.com/articles/nrneurol.2017.104

Summary

27
Summary
▪ CVST is rare, but clinically serious, and can result in substantial morbidity and mortality;
not usually associated with thrombocytopenia
▪ Observed cases following Janssen COVID-19 vaccines appear to exceed expected based
on background rates of CVST among women aged 20–50 years (3-fold or greater)
‒ All 6 reports were in women age range 18–48 years, all with thrombocytopenia
‒ No obvious patterns of risk factors detected
▪ CVST with thrombocytopenia has not been observed after the two authorized mRNA
vaccines
‒ 182 million mRNA COVID-19 doses administered with no reported cases to date
▪ Clinical features of Janssen cases are similar to those observed following the
AstraZeneca COVID-19 vaccine in Europe
▪ Both Janssen and AstraZeneca vaccines contain replication-incompetent adenoviral
vectors (human [Ad26.COV2.S] for Janssen and chimpanzee [ChAdOx1] for AstraZeneca)

28
Summary (cont.)
▪ For clinicians
‒ Maintain a high index of suspicion for symptoms that might represent serious thrombotic events
or thrombocytopenia in patients who have recently received the Jansen COVID-19 vaccine,
including severe headache, backache, new neurologic symptoms, severe abdominal pain,
shortness of breath, leg swelling, petechiae (tiny red spots on the skin), or new or easy bruising.
Obtain platelet counts and screen for evidence of immune thrombotic thrombocytopenia.
‒ In patients with a thrombotic event and thrombocytopenia after the Jansen COVID-19 vaccine,
evaluate initially with a screening PF4 enzyme-linked immunosorbent (ELISA) assay as would be
performed for autoimmune HIT. Consultation with a hematologist is strongly recommended.
‒ Do not treat patients with thrombotic events and thrombocytopenia following receipt of Janssen
COVID-19 vaccine with heparin, unless HIT testing is negative.
‒ If HIT testing is positive or unable to be performed in patient with thrombotic events and
thrombocytopenia following receipt of Jansen COVID-19 vaccine, non-heparin anticoagulants and
high-dose intravenous immune globulin should be strongly considered.
‒ Report adverse events to VAERS, including serious and life-threatening adverse events and deaths
in patients following receipt of COVID-19 vaccines as required under the Emergency Use
Authorizations for COVID-19 vaccines.

29
Summary (cont.)
▪ For public health
‒ Encourage healthcare providers and the public to report all serious and life-threatening adverse
events and deaths following receipt of COVID-19 vaccines to VAERS as required under the EUAs
for COVID-19 vaccines.
‒ Disseminate information to healthcare providers in your jurisdictions.
▪ For the public
‒ If you have received the Janssen COVID-19 vaccine and develop severe headache, abdominal
pain, leg pain, or shortness of breath within three weeks after vaccination, contact your
healthcare provider, or seek medical care.
‒ Report adverse events following receipt of any COVID-19 vaccine to VAERS.
‒ If you are scheduled to receive the Janssen vaccine, please contact your healthcare provider,
vaccination location, or clinic to learn about additional vaccine availability.

30
How to report an adverse event to VAERS
▪ Go to vaers.hhs.gov
▪ Submit a report online
▪ For help:
Call 1-800-822-7967
Email info@VAERS.org
video instructions
https://youtu.be/sbCWhcQADFE
▪ Please send records to VAERS
ASAP if contacted and asked
‒ HIPAA permits reporting of
protected health information
to public health authorities
including CDC and FDA

31
Next steps
▪ Continue enhanced monitoring in VAERS and other vaccine safety
systems (e.g., Vaccine Safety Datalink [VSD])
‒ VSD: ~113K Janssen doses administered, 0 cases in risk interval(s)
▪ Investigate potential cases through detailed clinical reviews/chart reviews
▪ Refine analyses to better quantify risk

32
Acknowledgments
Centers for Disease Control and Prevention
COVID-19 Vaccine Task Force
COVID-19 Vaccine Task Force, Vaccine Safety Team
Immunization Safety Office
Division of Healthcare Quality Promotion
Clinical Immunization Safety Assessment Project
Vaccine Adverse Event Safety Network
We wish to acknowledge the contributions of investigators from the following organizations:

33
Additional report of patient with non-CVST thromboses and
thrombocytopenia after Janssen COVID-19 vaccine*
▪ 50s y/o female
▪ History coronary artery disease, hypertension, asthma, COPD
▪ Developed bruising and leg swelling 11 days after vaccination with
Janssen vaccine
▪ Hospitalized with hematologic event that is non-CVST
‒ Left lower extremity deep venous thrombosis
‒ Right superficial femoral artery and bilateral iliac artery thrombosis (non-CVST)
▪ Thrombocytopenia of 15,000/mm
3
*Assessment based only on VAERS report; investigation in-progress including obtaining and reviewing medical records
For more information: www.cdc.gov/COVID19
Thrombocytopenic thrombosis after
Janssen vaccine
COVID-19 Vaccines
Sara Oliver MD, MSPH
COCA Call
April 15, 2021
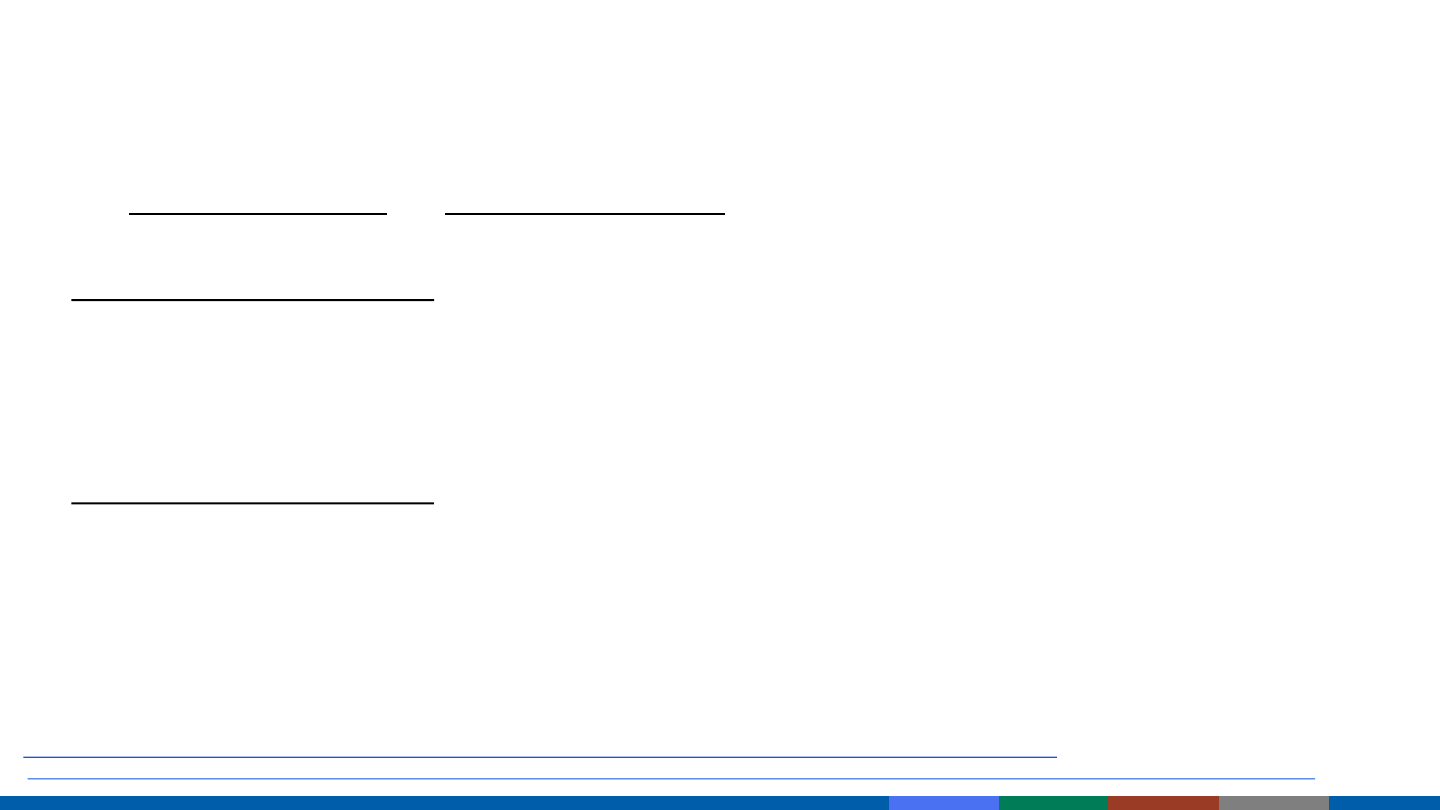
Adenovirus vector vaccines
35
▪ Concerns for rare clotting events seen after COVID-19 adenovirus vector vaccines
▪ Clinical syndromes after both vaccines appear similar
▪ However, extent to which the cases seen after both adenovirus vector vaccines
represent the same syndrome is unknown
Adenovirus Vector
Janssen/J&J
AstraZeneca
Janssen
One dose
Human Adenovirus 26 vector
EUA in the US issued Feb 2021
EMA authorized for Europe
Doses not yet
delivered/administered
AstraZeneca
Two doses
Chimp adenovirus vector
Awaiting EUA application in the US
Approved in UK, Europe
EUA: Emergency Use Authorization; EMA: European Medicines Agency
▪ Last week, EMA’s safety committee (PRAC) released report concluding:
– Strong association and probable causal link between the AZ vaccine and rare clotting events
From the European Union:
▪ 62 cases of CVST & 24 cases of splanchnic vein thrombosis with thrombocytopenia; 18 were fatal
▪ Most in females <60 years of age
▪ Within 2 weeks of AZ vaccine receipt
▪ Due to different ways vaccine used in each country, cannot exclude age/gender as risk factors
From the United Kingdom:
▪ 79 cases of thrombosis + thrombocytopenia; 19 were fatal
▪ 44 cases of CVST (14 fatalities) & 35 cases of other clots (5 fatalities)
▪ 51 cases were female; 28 were male
▪ 20.2 million doses given. Estimated risk ~4 per million pop. (‘slightly higher incidence’ in younger age groups)
36
AstraZeneca (AZ) vaccine
https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood
https://www.gov.uk/government/publications/use-of-the-astrazeneca-covid-19-vaccine-jcvi-statement/jcvi-statement-on-use-of-the-astrazeneca-covid-19-vaccine-7-april-2021
CVST: Cerebral Venous Sinus Thrombosis

Reports of low platelets (thrombocytopenia) and blood clots (thrombosis) after AZ vaccine in Europe
37
Vaccine-induced immune thrombotic thrombocytopenia
Two publications describing
cases of thrombotic
thrombocytopenia from
Germany & Austria, and
Norway
Many cases had platelet
activating antibodies
directed against platelet
factor 4 (PF4)
Authors propose syndrome
entitled “Vaccine-induced
immune thrombotic
thrombocytopenia” (VITT)
https://www.nejm.org/doi/full/10.1056/NEJMoa2104840?query=featured_hom
e
https://www.nejm.org/doi/full/10.1056/NEJMoa2104882?query=featured_home

AstraZeneca (AZ) vaccine:
Recommendations for use
38
▪ EMA's Pharmacovigilance Risk Assessment Committee (PRAC) does not make
vaccine policy for the EU; each country weighs the risks and benefits of AZ
vaccine individually
▪ Many countries have adopted age-based recommendations
– UK: Adults ≥30 years of age; April 7, 2021
– Australia: Adults ≥50 years of age; April 8, 2021
– Other European countries: Adults ≥55 to ≥70 years of age
EMA: European Medicines Agency
39
Discussion by the Work Group
▪ Benefit/risk balance for use of the Janssen COVID-19 vaccine
▪ Review of cerebral venous sinus thrombosis (CVST) cases
▪ Risk of COVID-19 disease, by sex and age
▪ COVID-19 vaccines administered, by age
▪ Janssen vaccine doses administered to date
▪ Projected supply of COVID-19 vaccines in the US
▪ Policy options for updated recommendations for use for Janssen COVID-19
vaccine

40
CVST cases reviewed by the Work Group
▪ 6 cases of CVST reported to VAERS
– All 6 among women 18-48 years of age
– Interval from vaccine receipt to symptom onset ranged from 6-13 days
▪ 1 case of CVST reported in the Phase 3 clinical trial
– 25-year-old male, no previous medical history, no medications
– Day 9 after vaccination: fever, headache
– Day 19 after vaccination: seizure, CT with cerebral hemorrhage
Day 21 after vaccination: CVST diagnosed, anti-PF4 positive
HAN Archive - 00442 | Health Alert Network (HAN) (cdc.gov) https://emergency.cdc.gov/han/2021/han00442.asp
CVST: Cerebral Venous Sinus Thrombosis
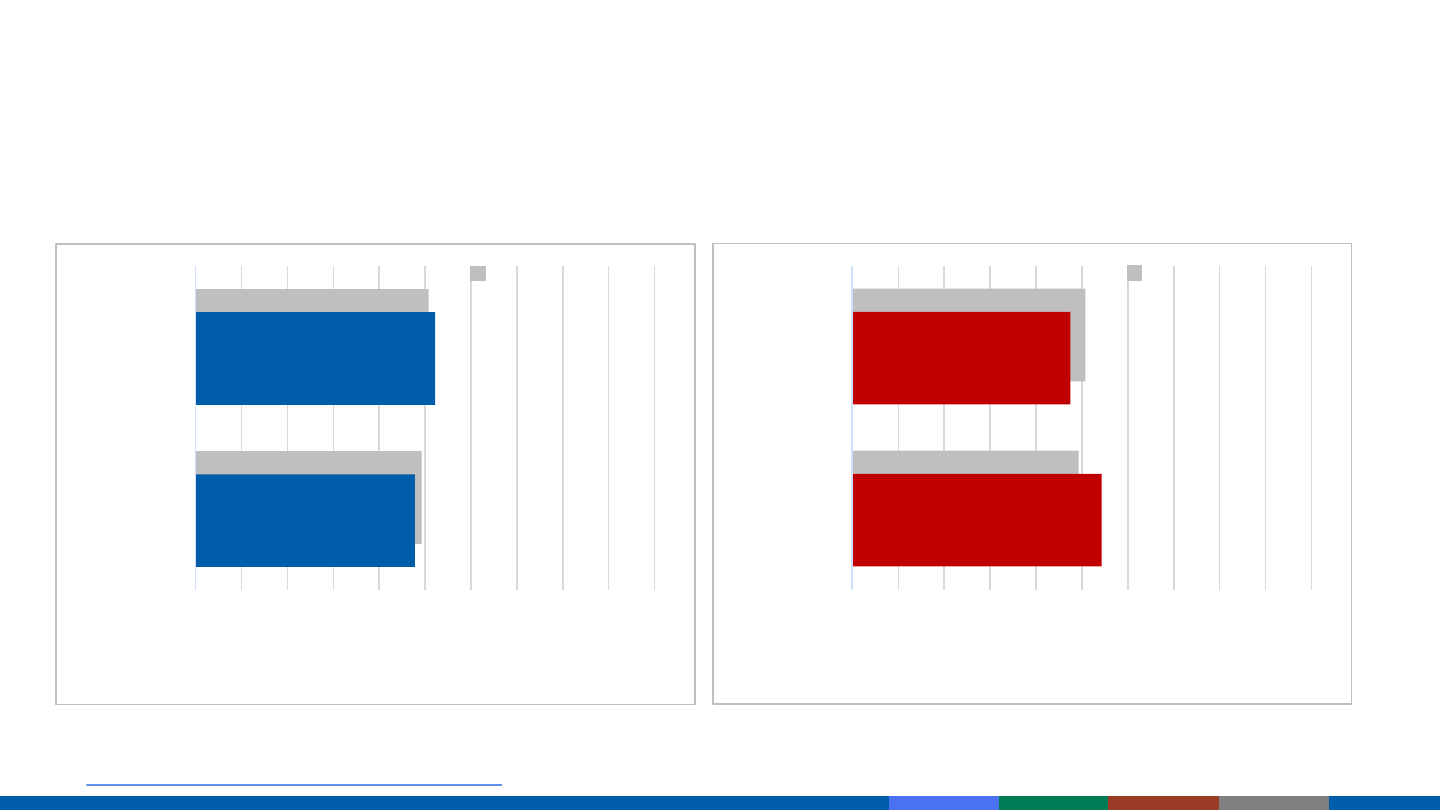
COVID-19 Cases and Deaths by Sex
https://covid.cdc.gov/covid-data-tracker/#demographics
COVID-19 Cases by Sex,
January 22, 2020 – April 12, 2021
COVID-19 Deaths by Sex,
January 22, 2020 – April 12, 2021
*Data from 24,349,551 cases, sex was available for 24,071,425
*Data from 433,171 deaths, sex was available for 432,059
4
0 10 20 30 40 50 60 70 80 90 100
Female
Male
Percent
Percent of US…
0 10 20 30 40 50 60 70 80 90 100
Female
Male
Percent
Percent of US…
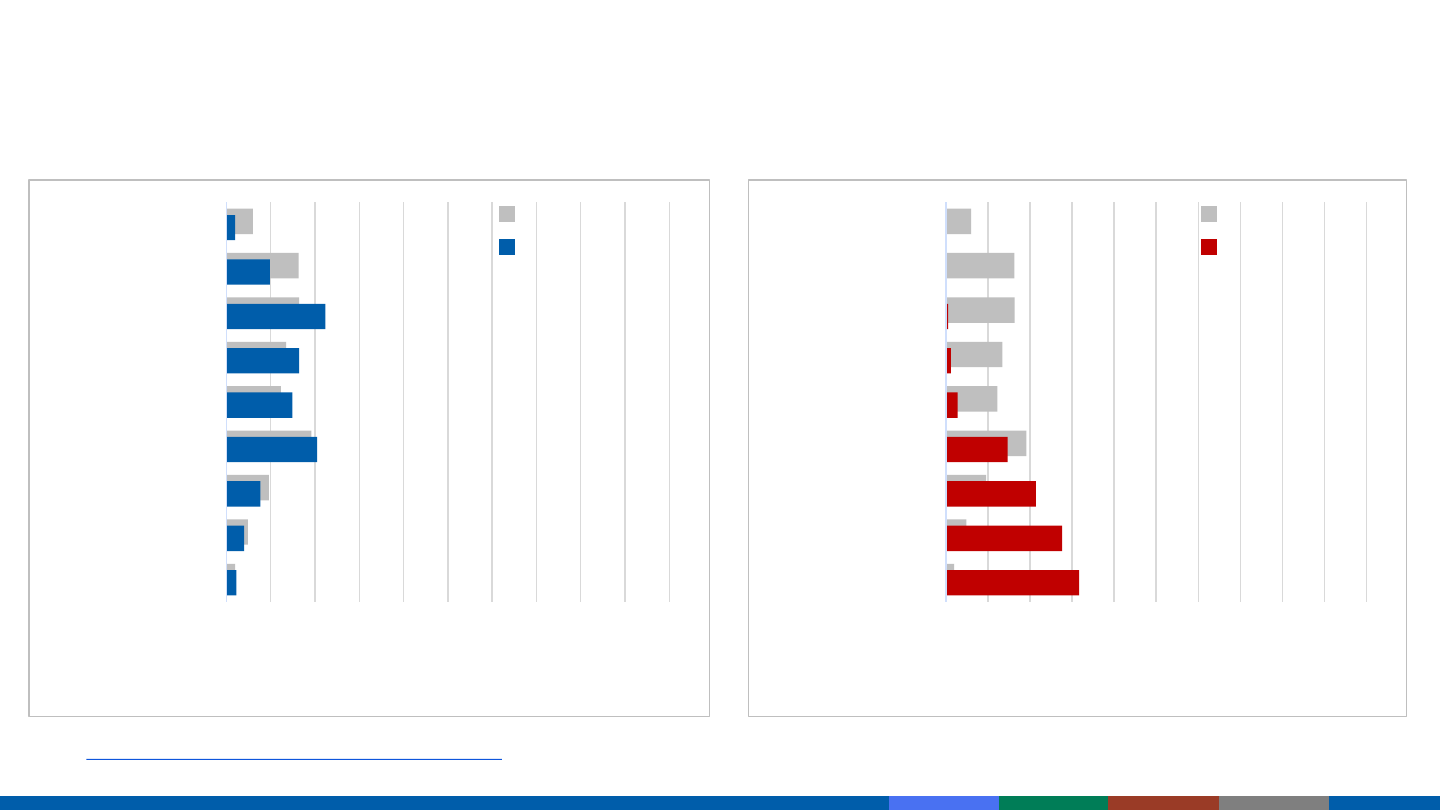
COVID-19 Cases and Deaths by Age Group
https://covid.cdc.gov/covid-data-tracker/#demographics
Data from 24,349,551 cases. Age group was available for 24,176,192
COVID-19 Cases by Age Group
January 22, 2020 – April 12, 2021
5
0 10 20 30 40 50 60 70 80 90 100
0-4 Years
5-17 Years
18-29 Years
30-39 Years
40-49 Years
50-64 Years
65-74 Years
75-84 Years
85+ Years
Percent
Percent of…
Percent of…
0 10 20 30 40 50 60 70 80 90 100
0-4 Years
5-17 Years
18-29 Years
30-39 Years
40-49 Years
50-64 Years
65-74 Years
75-84 Years
85+ Years
Percent
Percent of…
Percent of…
COVID-19 Deaths by Age Group
January 22, 2020 – April 12, 2021
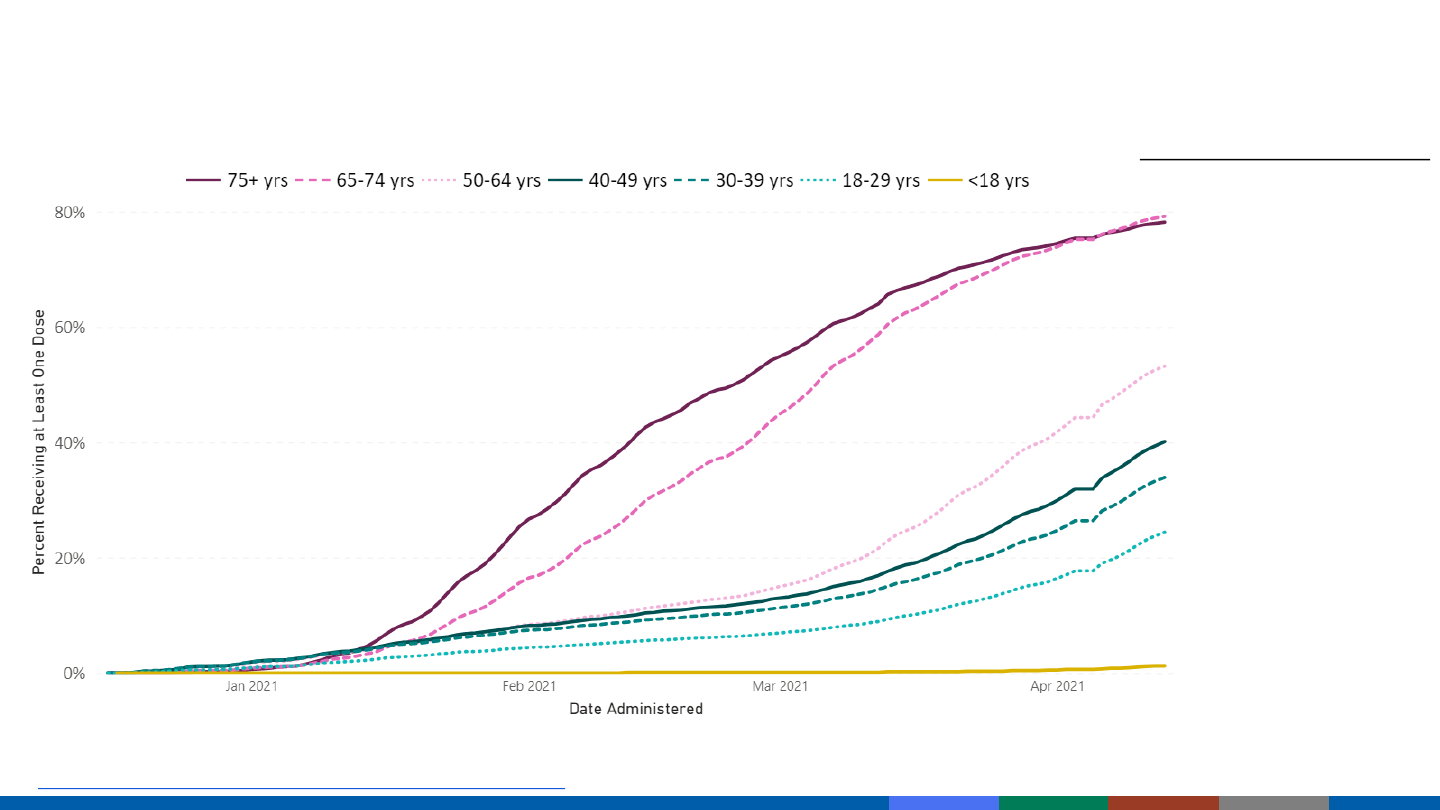
COVID-19 Vaccination Coverage by Age – United States
Data as of April 13, 2021; age available for 92% of doses administered.
https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic
10
Percent Receiving ≥1 dose
≥75 years 78.2%
65-74 years 79.2%
50-64 years 53.2%
40-49 years 40.1%
30-39 years
33.9%
18-29 years
24.4%
▪ 7,233,726 Janssen doses administered to date
– 1,495,400 Janssen doses administered to females 18-50 years of age*
10
Janssen Doses Administered to Date
Source: CDC Immunization Data Lake; Includes data reported to CDC as of 4/13/2021 at 6:00 am
*Data stratified by age and sex does not include Texas.
12
Janssen Doses Administered to Date
Source: CDC Immunization Data Lake; Includes data reported to CDC as of 4/13/2021 at 6:00 am
*Data stratified by age and sex does not include Texas.
Prior to March 30:
March 30 to April 13
3,466,166 Janssen doses administered
48% of doses
3,767,560 Janssen doses administered
52% of doses
Thrombocytopenic thrombotic events develop ~6-13 days after vaccine receipt
Thrombocytopenic
thrombotic events post-
vaccine likely already
occurred
Thrombocytopenic thrombotic
events post-vaccine may still
occur
7,233,726 doses administered in the United States
▪ Thrombocytopenic thrombotic events after the AstraZeneca vaccine have
occurred
▪ In the US, 6 cases of CVST reported after receipt of the Janssen COVID-19 vaccine.
▪ No cases of CVST with thrombocytopenia reported after receipt of either Pfizer
and Moderna COVID-19 vaccines
▪ CVST cases have occurred primarily in younger adults, females
▪ CVST can be clinically devastating or fatal
▪ In the US, alternative COVID-19 vaccines (mRNA vaccines) are available
– Based on current projections, supply of both mRNA vaccines fairly stable for near future
14
What is known so far
CVST: Cerebral Venous Sinus Thrombosis
▪ True background incidence of CVST with thrombocytopenia
▪ Specific risk factors for thrombocytopenic thrombotic events
▪ Incidence of other thrombotic (non-CVST) cases with thrombocytopenia after
Janssen vaccine
▪ Ability to compare or generalize thrombotic cases after the AstraZeneca
vaccine to Janssen vaccine
▪ True incidence of thrombocytopenic thrombotic events/CVST after a
Janssen/J&J COVID-19 vaccine
– More cases may be identified in the coming days/weeks
15
What we do NOT know
CVST: Cerebral Venous Sinus Thrombosis

Policy discussions from ACIP:
Janssen/J&J COVID-19 vaccine
▪ While overall reported cases are rare, once limited to doses administered to age
and sex of CVST cases seen, observed cases exceed expected cases
▪ Given the timing of doses administered (52% of doses administered in the previous
2 weeks), additional cases may be identified over the next 1-2 weeks
▪ Emphasis that robust safety surveillance is critical
– Signal detection and evaluation of cases occurred as planned
17
Policy options: Janssen/J&J COVID-19 vaccines
WG Discussion points
– Adults 50 years of age and older only
– Males only
19
Policy Options for Janssen Policy Recommendations
Age or gender
specific
populations?
Do not recommend
use of Janssen
vaccine
Recommend use of
Janssen/J&J COVID-19
vaccine in all adults
≥18 years of age
Recommend use of
Janssen/J&J COVID-19
vaccine in some
populations

▪ Monday 4/12: Vaccine Safety Technical Group (VaST) meeting
▪ Tuesday 4/13: ACIP COVID-19 vaccines Work Group meeting
▪ Wednesday 4/14: Emergency ACIP meeting
▪ Consider implications of reported cases of thrombosis and
thrombocytopenia after Janssen/J&J vaccine on vaccination policy
Janssen/J&J COVID-19 vaccine:
ACIP Response
51
Purpose of Emergency ACIP meeting

52
Janssen/J&J COVID-19 vaccine:
HAN released April 13, 2021
HAN Archive - 00442 | Health Alert Network (HAN) (cdc.gov) https://emergency.cdc.gov/han/2021/han00442.asp
▪ Recommendations for Clinicians: diagnosis and treatment
– Evaluate patients with a screening PF4 enzyme-linked immunosorbent (ELISA) assay as would be performed for
autoimmune HIT. Consultation with a hematologist is strongly recommended.
– Do not treat with heparin, unless HIT testing is negative
▪ Recommendations for Public Health: case reporting through VAERS
– Encourage healthcare providers and the public to report all serious and life-threatening adverse events and
deaths following receipt of COVID-19 vaccines to VAERS
▪ Recommendations for the Public: clinical signs and symptoms to monitor
– Contact healthcare provider, or seek medical care if you develop severe headache, abdominal pain, leg pain, or
shortness of breath within three weeks after vaccination with the J&J COVID-19 vaccine

For more information, contact CDC
1-800-CDC-INFO (232-4636)
TTY: 1-888-232-6348 www.cdc.gov
The findings and conclusions in this report are those of the authors and do not necessarily represent the
official position of the Centers for Disease Control and Prevention.
Thank you

To Ask a Question
▪ Using the Zoom Webinar System
– Click on the “Q&A” button.
– Type your question in the “Q&A” box.
– Submit your question.
▪ If you are a member of the media, please direct your questions to
CDC Media Relations at 404-639-3286 or email [email protected].
▪ If you are a patient, please refer your question to your healthcare provider.
Upcoming COCA Calls / Additional COVID-19 Resources
– Subscribe to receive notifications about upcoming COCA calls and other COCA
products and services at emergency.cdc.gov/coca/subscribe.asp
– Share call announcements with colleagues
– Sign up to receive weekly COVID-19 Science Updates
by visiting cdc.gov/library/covid19/scienceupdates.html?Sort=Date%3A%3Adesc

COCA Products & Services
COCA Call Announcements contain all information
subscribers need to participate in COCA Calls. COCA
Calls are held as needed.
Monthly newsletter that provides information on CDC
training opportunities, conference and training
resources, the COCA Partner Spotlight, and the
Clinician Corner.
As-needed messages that provide specific, immediate
action clinicians should take. Contains comprehensive
CDC guidance so clinicians can easily follow
recommended actions.

COCA Products & Services
Informs clinicians of new CDC resources and guidance
related to emergency preparedness and response.
This email is sent as soon as possible after CDC
publishes new content.
CDC's primary method of sharing information about
urgent public health incidents with public information
officers; federal, state, territorial, and local public
health practitioners; clinicians; and public health
laboratories.
Monthly newsletter providing updates on emergency
preparedness and response topics, emerging public
health threat literature, resources for health
professionals, and additional information important
during public health emergencies and disasters.

Join Us On Facebook!






